
Comparisons of results show that the currently published correlations give poor estimates of K-values for all components, while the proposed new model improved significantly the average absolute deviation error for all components. These K-values were then used to build the model using the Discipulus software, a commercial Genetic Programming system, and the results of K-values were compared with the values obtained from published correlations. Material balance techniques were used to extract the K-values of crude oil and gas components from the constant volume depletion and differential liberation tests for the oil and gas samples, respectively. Scribd is the worlds largest social reading and publishing site.

none of the original adjustments used by De Priester in his im.
#Depriester chart pdf pdf#
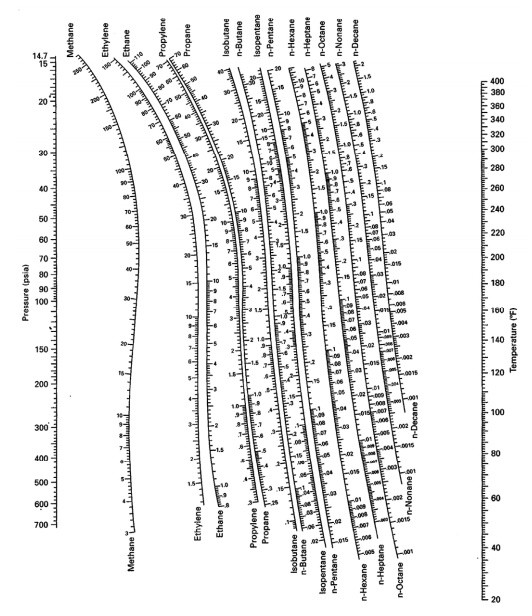
Constant Volume Depletion (CVD) and Differential Liberation (DL) were conducted for these samples. De Priester Chart - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Charts of vapor-liquid equilibrium K-values for hydro- carQns were prepared from f/P vs. In this paper, 732 high-pressure K-values obtained from PVT analysis of 17 crude oil and gas samples from a number of petroleum reservoirs in Arabian Gulf are used. The new model is applied to multicomponent mixtures. We will require to change a caption of our chart. This paper presents a new model for predicting K values with genetic programming (GP). 0 Comments You can consider to make use of a customization right here: Its a good XSL-transformation utilized to get these charts.Here is definitely a list of values for this paraméter. Several techniques are available in the literature to estimate the K-values. temperature composition, so that relations for K i can be fit by experimental data and used directly or in the form charts. In particular, they are critical for reliable and successful compositional reservoir simulation. They are important in predicting compositional changes under varying temperatures and pressures in the reservoirs, surface separators, and production and transportation facilities. ~.Equilibrium ratios play a fundamental role in understanding the phase behavior of hydrocarbon mixtures. If the liquid is 0.10 mole fraction h-butane, find the compositions of Iiquid and vapor. a)ĭew Point Calculation T (0 C)= T (0 R)= KĪ mixture of n-butane, n-pentane, and n-hexane is at 120° F and 20 psia. Use the aEP value to generate the y-x equilibrium diagram.įind the dew-point and bubble-point temperatures for a mixture that is 20 mole ~o n-butane, 50 mole % n-pentane, and 30 mole % n-hexane. Tutorials 2 and 4 Questions.pdf Exam questions and answers.pdf Summary The Students Guide to Cognitive Neuroscience lectures 1-13 Exam 2012. A t 1 atm, the ethylene dibromide-propy lene dibromide system has a constant relative volatility of a= 1.30 (Perry et al., 1963, p. When 80% has been vaporized, what iS the temperature and what are the liquid and vapor compositions?ĭ1l. K (or DePriester) Chart (high T range) in American Engineering Units from Introduction to Chemical Engineering Thermodynamics (7th ed) by Smith, J.M., Van.

At what temperature would it stop boiling (assume no material is removed)? What is the composition of the last dropIet of liquid? c. At what temperature does it first begin to boil? What is the composition of the first bubble of vapor? b. If a 40 mole % ethanol, 60 mole % water mixture at 60° C and 1 atm is heated: -Ī. Use the DePriester chart to generate the temperature-composition diagram for isobutane and propane at 1000 kPa.


 0 kommentar(er)
0 kommentar(er)
